Background. The Revised International Staging system (R-ISS) is the standard risk stratification model used for newly diagnosed (ND) multiple myeloma (MM) (Palumbo et al. JCO 2015). R-ISS identifies 3 groups of patients (pts) with different PFS and OS. However, 60% of pts are considered as intermediate-risk (R-ISS II), possibly including pts with different risk of progression/death. Recently, 1q copy number alterations (CNAs), which were not included in the R-ISS, proved to be a poor prognostic factor in NDMM pts.
The European Myeloma Network (EMN), under the umbrella of the HARMONY project, collected more than 7000 patient data from European clinical trials. The aim of this analysis is to revise the R-ISS risk stratification model, by analyzing the prognostic value of each single baseline risk feature, including 1q CNAs, to improve prognostication in NDMM pts.
Methods. Data from 15 European clinical trials enrolling NDMM pts from 2005 to 2014 were collected through EMN and registered in HARMONY platform. HARMONY is a European public-private partnership focusing on hematologic malignancies with unmet medical needs, including MM. OMOP Common Data Model was used to harmonize data. All pts received an immunomodulatory agent (IMiD) and/or a proteasome inhibitor (PI) upfront. In a multivariate Cox regression analysis adjusted for age, sex and therapy, we evaluated the impact of each risk feature on overall survival (OS) and progression-free survival (PFS). The hazard of death conferred by the most significant variables was used to create an additive risk score.
Results. 7077 NDMM pts were registered in the HARMONY platform and included in the analysis. Data were mature with a median follow-up of 75 months; median age was 62 years. The majority of pts were transplant-eligible (65%). 40% of the pts received IMiDs only, 15% PIs only, 46% both drug classes during their first-line treatment.
In a multivariate Cox model, ISS (II vs I HR 1.55 p<0.001, III vs I HR 2.02 p<0.001), del(17p) (HR 1.74, p<0.001), LDH (HR 1.65, p<0.001), t(4;14) (HR 1.56, p<0.001) and 1q CNAs (HR 1.45, p<0.001) had the highest impact on OS. ISS (II vs I HR 1.35 p<0.001, III vs I HR 1.53 p<0.001), t(4;14) (HR 1.49, p<0.001), del(17p) (HR 1.41, p<0.001), 1q CNAs (HR 1.37, p<0.001) and LDH (HR 1.33, p<0.001) had the most remarkable impact on PFS. t(14;16) had a significant effect on OS (HR 1.34, p=0.006) but not on PFS (HR 1.15, p=0.13); thus, it was not included in the model.
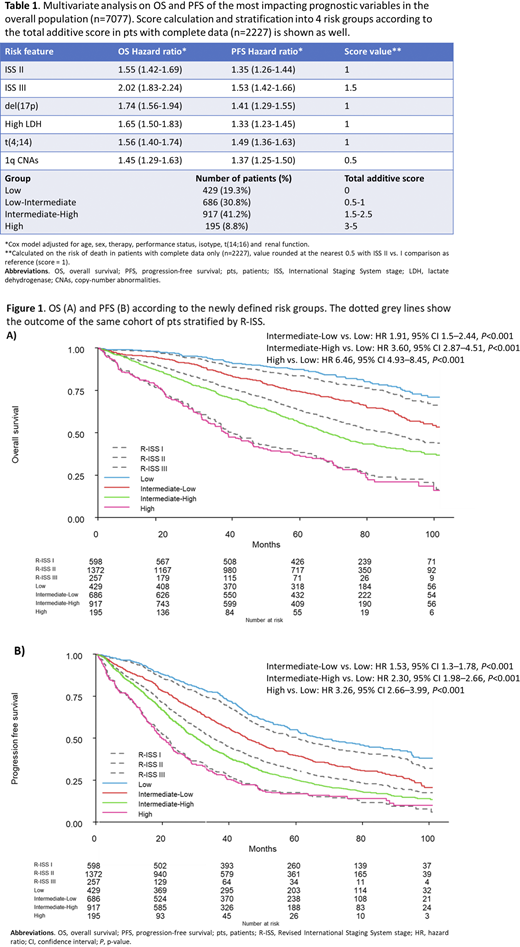
These prognostic variables were simultaneously present in 2227 pts and the most frequent reason of exclusion of the remaining pts was 1q CNAs that was missing in some of the trials. We exploited the OS impact of these risk features in pts with complete data to create an additive scoring system (Table 1).
Pts were then stratified into 4 groups: Low [(n=429 (19.3%), score 0)], Low-Intermediate [(n=686 (30.8%), score 0.5-1], Intermediate-High [(n=917 (41.2%), score 1.5-2.5] and High [(n=195 (8.8%), score 3-5]. Each group showed significantly different OS (Figure 1A) and PFS (Figure 1B).
Median OS was not reached vs 109.2 vs 68.5 vs 37.9 months and median PFS was 68 vs 45.5 vs 30.2 vs 19.9 months in the above 4 risk groups, respectively.
With this new stratification model, R-ISS stage II pts (n=1372) were better distributed into Low-Intermediate (n=517), Intermediate-High (n=811) and High risk (n=44) groups, confirming that this wide group included heterogeneous pts with a different risk of progression and/or death.
This new risk stratification maintained its prognostic value in subgroup analysis of transplant-eligible and transplant-ineligible pts and in pts receiving IMiDs, PIs or both.
Conclusion. This analysis on a large number of patient data collected thanks to a well-established European collaboration demonstrated that the existing R-ISS stratification model may be improved.
This additive scoring system based on the impact of single risk features could be the future risk stratification model for NDMM, so called "R2-ISS".
About half of the pts can be classified as Low or Low-Intermediate risk and about half of the pts can be classified as Intermediate-High or High risk, representing an opportunity to design risk-adapted approaches in a meaningful number of pts.
Moreover, such additive risk score easily allows the inclusion of new prognostic variables in the future as they continue to emerge. The inclusion of new patient data is ongoing, and validation in an independent cohort is planned.
D'Agostino:GSK: Membership on an entity's Board of Directors or advisory committees. Waage:Janssen: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy; Shire: Honoraria. Zamagni:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Takeda: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Speakers Bureau. Mateos:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Honoraria; PharmaMar-Zeltia: Consultancy; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie/Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Larocca:Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria. van de Donk:Takeda: Other: Ad Board; Genentech: Other: Ad Board; Bayer: Other: Ad Board; BMS: Other: Ad Board, Research Funding; Amgen: Other: Ad Board, Research Funding; Celgene: Other: Ad Board, Research Funding; Novartis: Other: Ad Board; Janssen: Other: Ad Board, Research Funding. Cairns:Celgene, Amgen, Merck: Research Funding; Celgene: Other: Travel Support. Salwender:Takeda: Honoraria; Bristol-Myers Squibb/Celgene: Honoraria; Janssen-Cilag: Honoraria; Amgen: Honoraria; Oncopeptides: Honoraria; Sanofi: Honoraria; GlaxoSmithKline: Honoraria; AbbVie: Honoraria. Blade Creixenti:Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Dürig:Janssen: Consultancy; AbbVie: Consultancy; Celgene: Consultancy. Bringhen:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Zweegman:Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cavo:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau; Karyopharm: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Goldschmidt:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product:, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product:, Research Funding; Dietmar-Hopp-Foundation: Other: Grants and/or provision of Investigational Medicinal Product:; Molecular Partners: Research Funding; Merck Sharp and Dohme (MSD): Research Funding; Novartis: Honoraria, Research Funding; Johns Hopkins University: Other: Grants and/or provision of Investigational Medicinal Product; Chugai: Honoraria, Other: Grants and/or provision of Investigational Medicinal Product:, Research Funding; Incyte: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product:, Research Funding; University Hospital Heidelberg, Internal Medicine V and National Center for Tumor Diseases (NCT), Heidelberg, Germany: Current Employment; GlaxoSmithKline (GSK): Honoraria; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants and/or provision of Investigational Medicinal Product, Research Funding; Mundipharma GmbH: Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding. Cook:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; IQVIA: Research Funding; Sanofi: Consultancy; Amgen: Consultancy; Roche: Consultancy; Karyopharm: Consultancy. San-Miguel:Amgen, BMS, Celgene, Janssen, MSD, Novartis, Takeda, Sanofi, Roche, Abbvie, GlaxoSmithKline and Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees. Boccadoro:Sanofi: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria; Mundipharma: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees. Sonneveld:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Skyline Dx: Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy.
The presentation includes discussion of off-label use of a drug or drugs for the treatment of multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal